Organization strategies for grant writing were discussed during Week 2. After reflection, please discuss your personal strategy for successful completion of the proposal you will be writing in this course.
The grant letter and the proposal letter is attached
An Introductory Summary of My Grant Proposal
1. Do you have an idea for a project? Is yes, what is it? If no, what area of your specialty interests you the most?
Yes. My research topic will be, “Avoiding failure in clinical trials.”
2. Is your project idea(s) compatible with your organization’s current mission and purpose? In what way? If no, can the idea be revised to better fit the organization’s current mission and purpose?
Yes, it is compatible with my organization’s current mission. This topic is compatible because the organization’s main aim is to carry out clinical trials and roll out to the market and ensure minimal failures, including minimizing adverse side effects to the subjects.
3. Is your project unique? Are there other organizations or groups doing this same work? Is there a way to revise the project idea to make it more unique?
My project is not unique. For example, Global Development Amgen-a biotechnology company focused on clinical research-has outlined what needs to be done to reduce clinical trials’ failures. I, however, used the word ‘entirely’ to mean that there is uniqueness based on how I intend to carry out my research and address unanswered questions despite there being existing topics. Additionally, I plan to share my research with adoption organizations. It is important to maintain a philosophy of continual improvement in clinical trials broadly and specifically to optimize every aspect of the development process (Fogel, 2018).
4. What need does your idea address? (The answer to this question will become the framework for the proposal’s need statement).
My idea addresses the tendency of researchers to rush to phase 3. This rush is a significant contributor to failures, as it renders researchers unable to review their progress and proactively anticipate any challenges adequately. Rushing studies into phase 3 after successful phase 2 trials may not provide time for reflection on how best to address safety in phase 3 (Fogel, 2018).
5. How would your idea improve the situation? (This answer will become the basis of the proposal’s goals and objectives).
Upon ensuring there is no rush to phase 3, I will have addressed the need for an exhaustive review of the previous phase. This will involve not only the research team but also external consultants to audit the project. As a result, any unidentified areas, inadvertently skipped objectives, tests, and other vital pointers will be identified and addressed proactively.
To boot, there will be a thorough review of the associated costs and any anticipated costs. Through this, necessary trials will not stall in the future due to funds exhaustion midway. Optimizing for cost by staging investment also slows down, while optimizing for speed tends to drive up the cost (Amgen Science, 2021). This means that a rush to phase 3 may achieve speed but yield more costs upon failure. Further, on successful completion of phase 3 because of a meticulously executed phase 2, there will be an increased likelihood of financiers’ willingness to fund future projects, hence creating a more successful research industry.
6. What do you plan to do to improve the situation? (This answer will become the basis of the proposal methodology).
To improve the situation, I will conduct a study of organizations with recorded failures in their previous research to obtain first-hand information on best and worst practices, consequences, and solutions, which I will share with all relevant bodies and regulators.
7. How will you know if your idea worked? (This answer will become the basis of the evaluation section of the proposal).
To know if my idea worked, I will evaluate the organizations’ and industry’s rate of new drugs’ approval before and after the implementation of my recommendations. The cost of success is so enormous because of all the money invested in drugs that fail: 90 percent of test drugs that enter clinical trials never get approved (Amgen Science, 2021). Into the bargain, I will review the availability of willing future financiers for the next phases, to determine the project’s potential for future advancement and industry-players’ support. Each researcher’s reputation and future grant funding depend on the success of each project (Halcomb, 2016).
8. How much will your idea cost? (This answer will become the basis of the budget). My idea is estimated to cost approximately USD. 100,000.00
9. Is there support within the organization for the project? Consider if the idea might need internal support of organization leadership/administration or perhaps external support from community leaders, school board, or church leaders.
Yes, the organization is supportive as the project will help address some of the industry’s most pressing challenges and reduce the rejection of new drugs’ rollouts. I am seeking approval from the local community and church leaders and local governments, as some of the data collection and trials may fall within their jurisdiction or violate individual rights given religious or traditional beliefs.
10. Is there a way in which these services might be funded in the future once the grant money runs out? (Think sustainability).
Yes, if the grant money runs out, the project can be funded by income generated from community-based initiatives that will be developed in the course of the research. Secondly, once the project has started, I will continuously search for additional grants by sharing our progress and success milestones to have new partners on board for emergencies.
References
Amgen Science. (2021). A Strategy for Making Clinical Trials More Successful. https://www.amgenscience.com/features/a-strategy-for-making-clinical-trials-more-successful/
Fogel, D. B. (2018). Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemporary clinical trials communications, 11, 156-164.
Halcomb, E. (2016). Measuring research success. Nurse Researcher.
Manager GrantWatch Company,
P O Box 45357
Stanford, California 92222
21st January 2021
Dear Sir/Madam
RE: SEEKING FINANCIAL AID FOR CLINIC PROJECT
Greetings, I hereby submit my proposal to the GrantWatch organization seeking funds in my project “Avoiding failure in clinical trials” This is a project-based in the department of health, the University of Texas in Rio Grande valley.
My project is purposefully geared towards exhibiting minimal clinical trial failures, minimizing clinical trials’ adverse effects on individuals, and ensuring risk-free clinical aftermath. The project is geared towards entirely eradicating clinical failures. Based on previous research and trials, there are lots of unanswered questions that my project would answer. The project would ensure maximum efficiency since it addresses issues such as; researchers rush to phase 3; this makes it difficult for them to analyze and evaluate potential challenges that would come henceforth. My project addresses how running into phase 3 after successful phase 2 trials may make it risky since the proper evaluation of risk-free phase 3 is minimized. This project costs $ 100,000
Successful phase 3 will lead to the project’s evaluation by a more sensitive and accurate team of experts. They’ll have to ensure no step is skipped or necessary procedures omitted. This will also include reviews from different individuals to ensure first-hand data from subjects themselves.
The project will take a maximum of three years of study, experiment, and research. It begins on January 30th of 2021 up to January 30th, 2023. Within this timeframe, it will be adequate to administer tests, experiments, evaluate results and get reviews and examinations from expatriates. My project is based at Rio Grande Valley, University of Texas, department of health sciences, Arlington TX, 76019-817-272-2011.
This project is necessary because it addresses a crucial aspect of clinical test analysis. It will create a more successful research industry for future clinical studies and experiments. This is because it addresses how to move from phases without error. This project’s main goals are to create a successful clinical administration concept of tests and experiments that don’t pose any risks to subjects. It’s also gearing towards creating a successful research industry that can help future researchers and financiers by providing a concrete concept.
I’m hopeful that my proposal will be highly regarded
Yours faithfully
Essay Writing Service Features
Our Experience
No matter how complex your assignment is, we can find the right professional for your specific task. Achiever Papers is an essay writing company that hires only the smartest minds to help you with your projects. Our expertise allows us to provide students with high-quality academic writing, editing & proofreading services.
Free Features
Free revision policy
$10Free bibliography & reference
$8Free title page
$8Free formatting
$8How Our Dissertation Writing Service Works
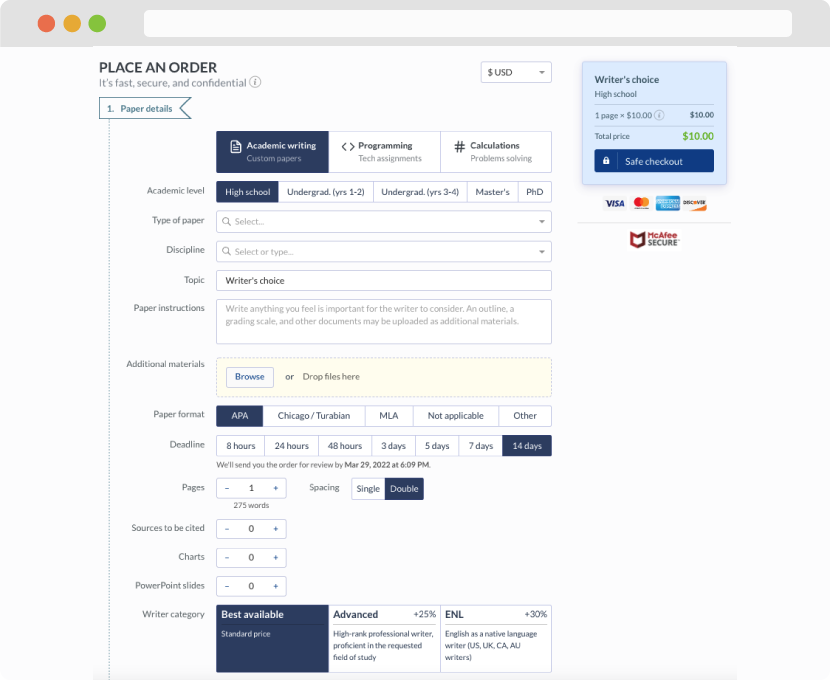
First, you will need to complete an order form. It's not difficult but, if anything is unclear, you may always chat with us so that we can guide you through it. On the order form, you will need to include some basic information concerning your order: subject, topic, number of pages, etc. We also encourage our clients to upload any relevant information or sources that will help.
Complete the order form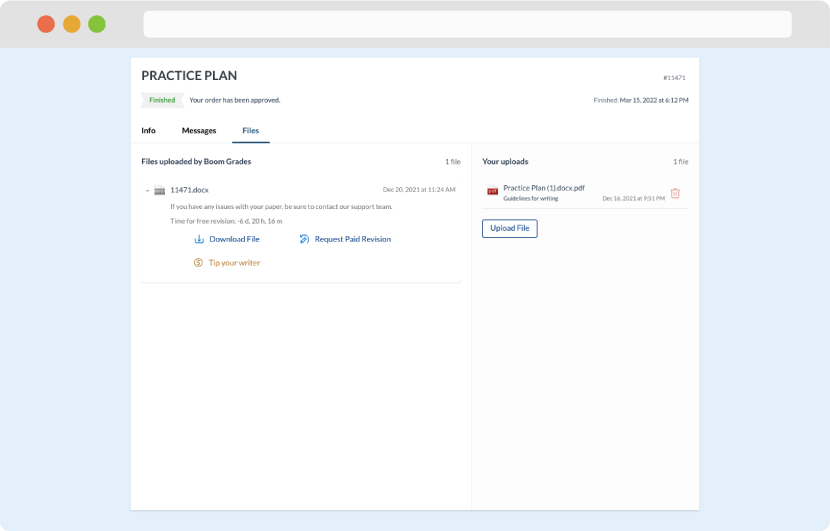
Once we have all the information and instructions that we need, we select the most suitable writer for your assignment. While everything seems to be clear, the writer, who has complete knowledge of the subject, may need clarification from you. It is at that point that you would receive a call or email from us.
Writer’s assignment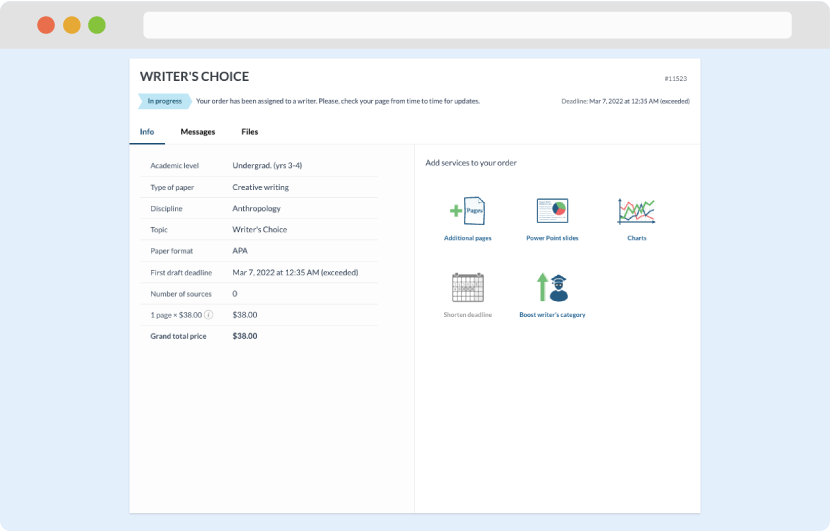
As soon as the writer has finished, it will be delivered both to the website and to your email address so that you will not miss it. If your deadline is close at hand, we will place a call to you to make sure that you receive the paper on time.
Completing the order and download